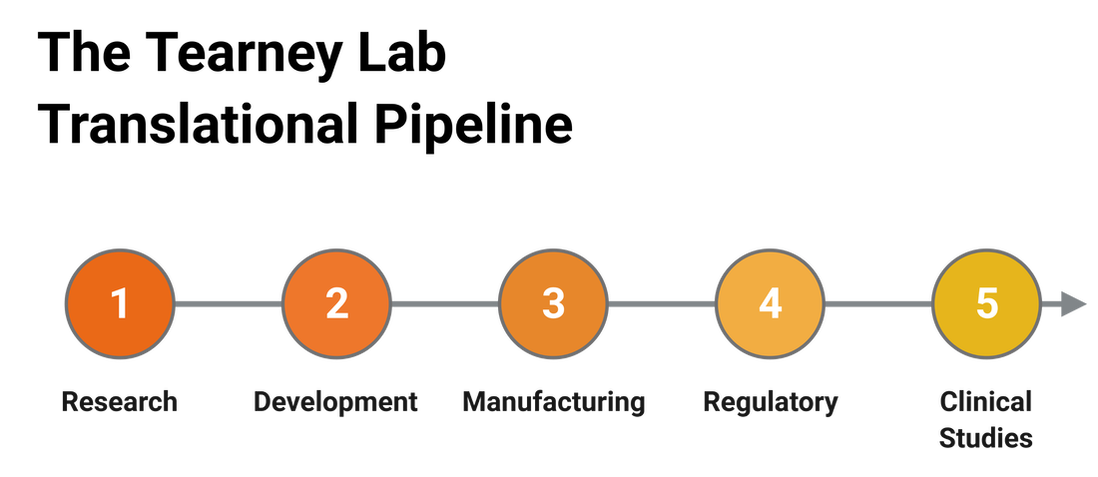
The Tearney Lab Translational Pipeline
The Tearney Lab is uniquely suited to conduct research that moves advanced technology from invention through fabrication, animal studies, regulatory approval, and clinical trials. This translational pipeline is unique in academia, integrating many concepts and processes that are more commonly found in industry. The output of this infrastructure allows all medical devices developed in the lab to be produced to a high quality standard with complete design history documentation. This level of advanced device development facilitates interactions with industry and accelerates the clinical translation of the imaging technologies developed in the lab.
Research
The Tearney Lab conducts optical research to invent and determine proof of concept of novel optical imaging technologies. Once a bench top instrument is fabricated, it is frequently validated using phantoms and animal and cadaver tissues to determine its potential clinical utility.
Development
Once a new imaging modality has been tested on the bench, it is transferred to an engineering team that designs, fabricates, and tests clinical prototypes.
Manufacturing
Once the prototype is fully validated, clinical-grade imaging systems and devices are manufactured by engineers and technicians in an advanced micro-machining facility and two clean rooms. All medical devices are designed using an industry-standard design control process.
Regulatory
The Tearney Lab has an experienced regulatory team that maintains device documentation and clinical inventory, interfaces with the institutional review board (IRB) and the FDA, and recruits patients for clinical studies.
Clinical Studies
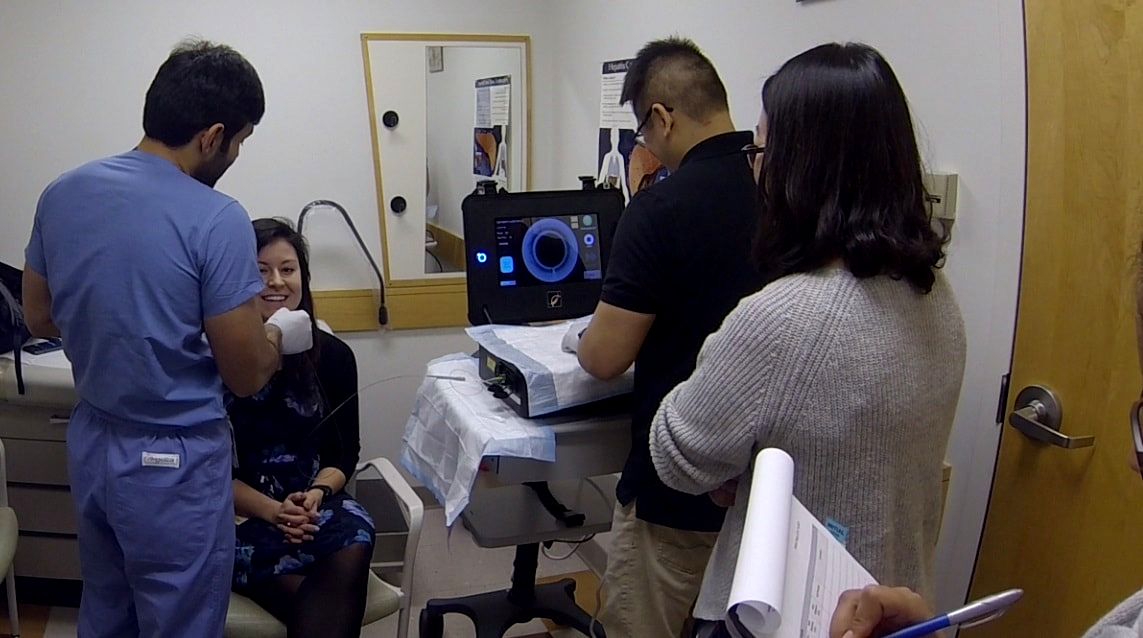
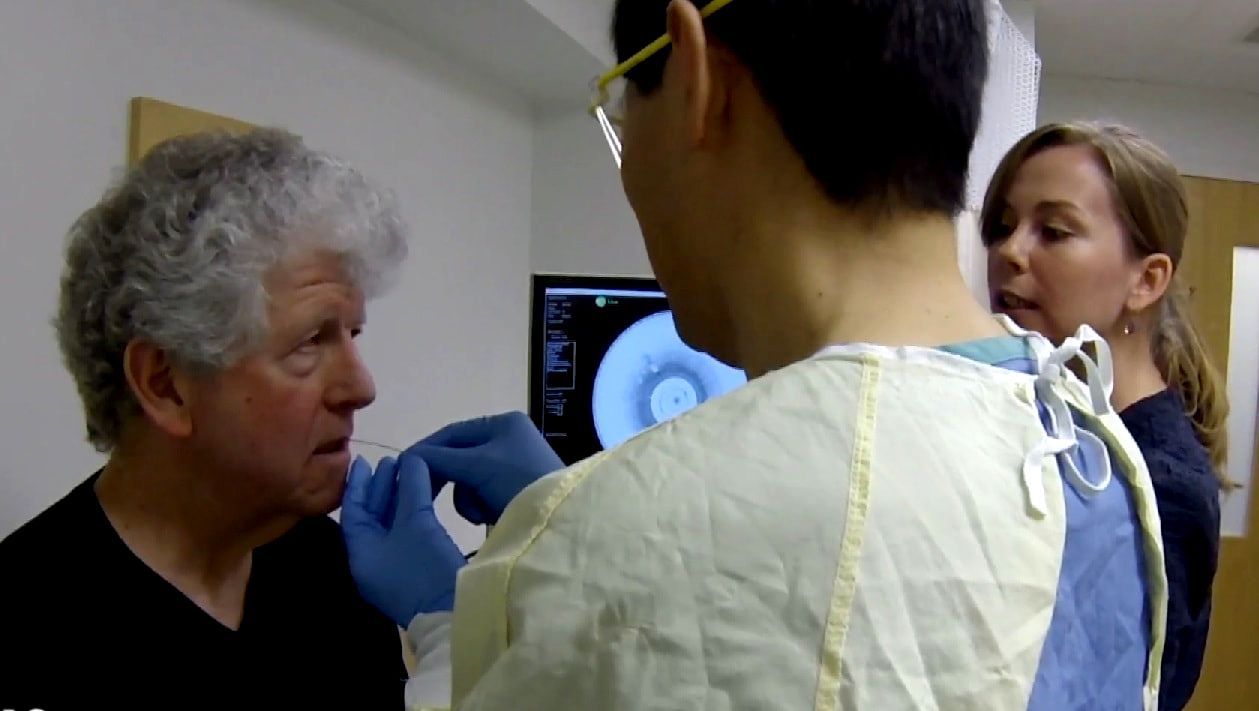
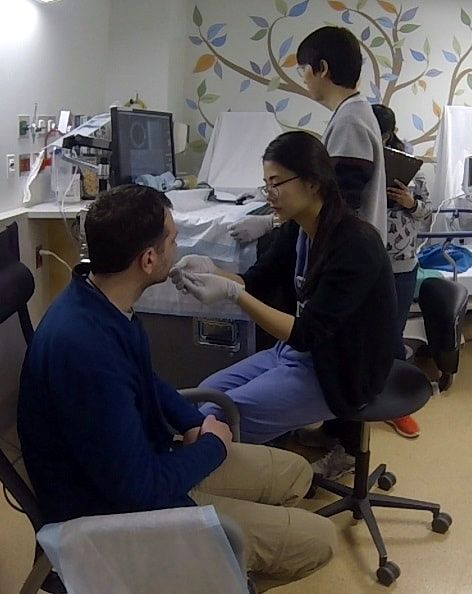
Approximately 10 single- and multi-center clinical studies are ongoing using different medical devices developed in the Tearney Lab. Each year several first-in-human studies are conducted as well as larger studies to validate or determine the efficacy of new imaging methods for solving particular medical problems.
Location, location, location.
Located on the Massachusetts General Hospital's main campus, the Tearney lab is able to execute very rapid translation of its devices into clinical trials and patient care settings.